Update on the State of Vaccines for Melanoma

Introduction
Scientists have recently advanced a strategy to send human cells instructions to generate an immune response against cancer. It is not an incredibly sophisticated strategy, but it is extremely logical. It takes advantage of the process cells use to communicate to the immune system about a problem. The difference is that we tell them what the problem is—cancer. If clinical trials continue to go well and show efficacy, we could see a vaccine approval to treat melanoma in a year or two.
Beginning with COVID-19
When the COVID-19 pandemic hit, a vaccine was desperately needed to stop the spread of the virus and save lives worldwide. Traditional vaccines would take time to produce and a more rapid solution was needed.
Fortunately, scientists, who would later win the Nobel Prize for their work, had been busy developing strategies for vaccine technology using molecules in the cell collectively called mRNA, or messenger ribonucleic acid, a relative to DNA. This relative, mRNA, takes the design plans from DNA, and conveys them to the cellular machinery that makes proteins.
Serendipitously, the science of mRNA was ideal for rapidly developing an mRNA-based vaccine to protect against COVID-19. In fact, several companies brought products to market—Moderna, Novavax, and Pfizer—by creating mRNA-based vaccines against the virus.
Although there may be ongoing concerns associated with requiring the COVID-19 vaccination, the incredible technology that helped eliminate the threat of dying from COVID has many other applications. Cancer researchers, who were already designing experimental therapies using mRNA, were paying very close attention to the safety and efficacy of these products in the marketplace. Now, some of their own ideas are being used in clinical trials against cancer.
Vaccines Designed to Prevent Disease
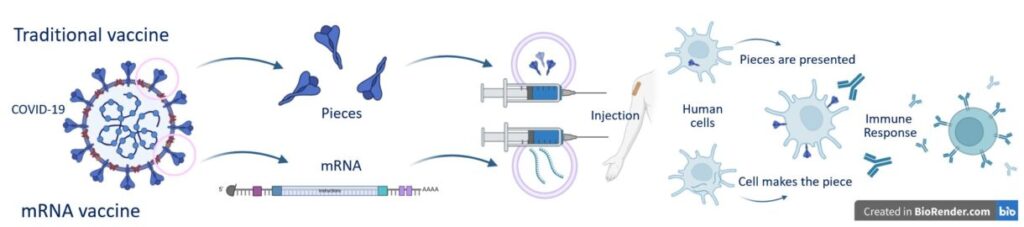
Traditional vaccination—the kind used to prevent disease—works by introducing a threat to your immune system and teaching your immune system to identify and fight that threat if it ever comes into contact with it. Many of the vaccines we receive as children are traditional vaccines.
COVID-19 vaccines are an example of this traditional technology combined with mRNA technology. mRNA sequences were used to create pieces of COVID-19 inside the human system, where they didn’t belong. The COVID-19 pieces would not be enough to recreate the entire virus—so no one would develop COVID-19 as a consequence of taking the vaccine—but these pieces would catch the immune system’s attention. Upon detection of the foreign COVID-19 pieces, the immune system developed
molecules to attack them. If someone later encountered COVID-19, the immune system would automatically recognize the foreign pieces and respond by fighting off the disease.
Cancer Vaccines Designed to Prevent Cancer
Similar to traditional vaccination, the idea behind cancer vaccines is to create instructions to teach your immune system to correctly identify a threat like a cancer cell and mount an immune response against it. However, teaching your immune system to distinguish a cancer cell from a normal one is challenging. In addition, the pieces of the cancer cell change–these are not the same from person to person, which makes developing these vaccines even more challenging.
We have had vaccines for many years that prevent certain cancers, but they are all cancers that are initiated by viral infection. Examples include the human papillomavirus (HPV) that causes cervical, anal, oral, penile, and other cancers; and hepatitis B infections that cause liver cancer. The vaccines for these cancers aren’t designed to help your immune system detect cancer cells; they’re designed to help your immune system detect and kill the viruses—HPV and hepatitis B—that can lead to cancer. At this time, we do not have any approved vaccines for cancer that enable your body to detect and kill a certain cancer cell.
There are currently no vaccines to prevent melanoma. Your best prevention comes from sun safety practices, especially if you have a light complexion.
Cancer Vaccines Designed to Treat Cancer
Cancer vaccines designed to treat cancer are a bit different. The individual already has cancer. The goal is to eliminate the cancer using the immune system.
The major challenge for this type of cancer vaccine is determining what pieces on cancer cells can be identified as unfamiliar—what part of a cancer cell will your immune system recognize as foreign and begin to fight? Unfortunately, because cancer cells develop within your body, they don’t generally appear to your immune system as foreign. And unlike the above noted cancers that stem from viruses,
there is no foreign virus to attack. Non-specifically training the immune system to attack your cells could cause a life-threatening immune reaction with long-term consequences.
However, using mRNA, scientists can tailor instructions to the immune system to specifically identify a cancer cell. If they exist, ideal targets would be anything the cancer cell uniquely expresses on the cancer cell surface or something it has in abundance compared to normal cells. In melanoma, several tumor-associated pieces (or “antigens”) are expressed in abundance by the melanoma cells, including Melan-A (melanoma antigen), MAGE-A3 (melanoma associated antigen 3), and NY-ESO-1 (New York esophageal squamous cell carcinoma 1).1
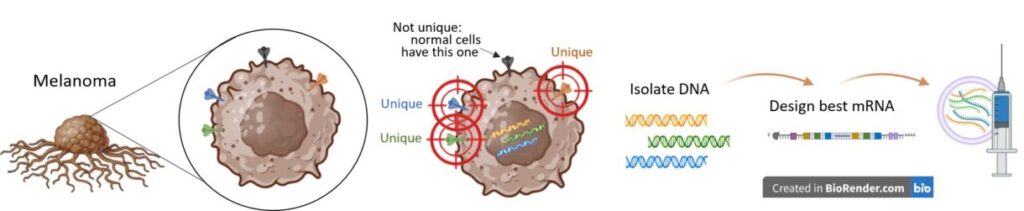
Each person’s cancer is likely to differ from the next. mRNA can create instructions for assembling pieces that cancer cells have but not normal cells. These are formally called “tumor-specific antigens.” Once the instructions, such as a cancer vaccine, are provided to the human system, normal cells will begin to make and present the cancer pieces to immune cells. If everything goes well, the immune system will respond by mounting an attack against cancer cells with those pieces.
One key difference between current traditional prevention vaccines and a cancer treatment vaccine is that the treatment vaccine can be personalized to help the patient’s immune system recognize the cancer in the patient’s body: The cancer cells can be taken from the own patient’s tumor or blood and then injected back into the patient with mRNA instructions to find and destroy those specific cancer cells.
Are Any mRNA Vaccines Approved for Melanoma?
No mRNA vaccines for melanoma have been approved by the FDA yet. However, several ongoing clinical trials are testing the safety and efficacy of vaccines to treat melanoma.
KEYNOTE-942: This active Phase II trial (NCT03897881) assesses mRNA-4157 (V940) plus pembrolizumab (Keytruda) in resected (surgically removed) high-risk Stage IIIB to Stage IV cutaneous melanoma. The mRNA used is custom-designed based on the individual’s tumor pieces (antigens) unique to their melanoma.
To make a personalized vaccine, a section of the tumor is removed to identify unique pieces or antigens. Once the unique pieces are identified, the instructions for 9 to 34 antigens will be created via mRNA. Then, the mRNA is injected back into the patient to train the immune system on what to target.
Data from the three-year trial update reported a 49% risk reduction in recurrence with mRNA-4157 plus pembrolizumab. Adding the mRNA vaccine also increased the 2.5-year recurrence-free survival rate from 55.6% to 74.8%. There was also a trend for improved overall survival to 96.0% versus 90.2% with pembrolizumab alone.2
A Phase III trial (NCT05933577) is ongoing for high-risk patients with melanoma to assess the safety and efficacy of mRNA-4157 (V940). It has enrolled 1089 patients with Stage IIB to Stage IV melanoma.3 Primary completion is expected in 2029, and full completion in 2030. The study has 165 locations worldwide.4
BNT111 mRNA Vaccine: This active Phase II trial (NCT04526899) is for “BNT111 and Cemiplimab in Combination or as Single Agents in Patients With Anti-PD-1-refractory/Relapsed, Unresectable Stage III or IV Melanoma.” It began in 2024 and is estimated to be completed in July 2026.5
BNT111 is a melanoma vaccine that contains mRNA against four tumor-associated pieces or antigens. These include MAGE-A3, NY-ESO-1, tyrosinase, and TPTE (transmembrane phosphatase with tensin homology). Many of these are expressed in abundance on the surface of melanoma cells but not normal cells. The company also included additional modifications within the mRNA design to enhance the successful identification of cancer cells by the immune cells.6 This vaccine is not personalized to each particular patient, but the mRNA is designed to identify specific antigens associated with melanoma.
STX-001 mRNA Vaccine: This Phase I/II trial (NCT06249048) is recruiting patients with advanced melanomas that can be injected, excluding uveal melanoma. U.S. sites included in the study are NextGen Oncology, the University of Pittsburg Medical Center, and the University of Texas M.D. Anderson Cancer Center. Patients included have received an immune checkpoint inhibitor therapy, such as ipilimumab (Yervoy), nivolumab, or pembrolizumab, either alone or in combination. They also must have either primary or secondary checkpoint inhibitor resistance.7
The mRNA created by STX-001 is interleukin-12 or IL-12. This molecule is displayed on the surface of specific white blood cells, called monocytes, that attract the attention of more effective, killing types of immune cells. If you were to have your blood analyzed and your monocytes were high, it could indicate the presence of an infection or cancer in your body.
When IL-12 is expressed by melanoma cells on their cell surface, it increases the presence of effective, killing immune cells. In addition, IL-12 reduces the expression of checkpoint controls that stop the immune system from fighting cancer. 8 This vaccine is not personalized to each patient, but specific to melanoma.
Multiple clinical trials have previously examined the use of IL-12 in melanoma. Results have been mixed but improved outcomes have been shown. Future clinical trials need to be conducted in combination with other anti-cancer agents since IL-12 reduces the targets of immune checkpoint inhibitors, making these drugs ineffective.8
When Will mRNA Vaccines be Available for Patients with Melanoma?
Although the technology is exciting, there are no approvals yet, and the approach has limitations. For one, personalizing the anti-cancer targets that should be included for each patient is challenging. The design process is resource-intensive and expensive. To adopt such an approach, the vaccine must have a significant benefit in treating the patient to justify these costs.
Ironically, the challenge of designing an appropriate mRNA vaccine is also its advantage. With flexibility in the design platform, scientists have created a way to vaccinate humans against nearly anything that our cells can build inside the body. The customization allows vaccine changes to fight cancer resistance to drugs and recurrent or indolent tumors.
Even though there are no approvals yet, in February 2025, a Phase II study using autogene cevumeran (RO7198457, RG6180) was completed in patients with previously untreated advanced melanoma (NCT03815058). The agent is an mRNA-based vaccine that creates personalized instructions to target specific pieces or antigens of the patient’s tumor. The results have not yet been released for melanoma. Still, a similar trial using autogene cevumeran in pancreatic cancer is ongoing and showing success.
Researchers in the pancreatic cancer trial used mRNAs targeting up to 20 pieces of antigens on the surface of cancer cells. After a year and a half, 50% of the vaccinated patients showed cells with evidence of the vaccine, and these individuals also had delayed recurrence of their cancer. After three years, the vaccine responders had not reached a measurement for recurrence-free survival (because most of them were doing well). In comparison, non-responders of the vaccine had a recurrence-free survival of 13.4 months.9
More clinical trials are required to determine whether mRNA vaccines are safe and effective for patients with melanoma. Sometimes, trials produce unclear results, or they can’t produce enough results. For example, a Phase I/II study (NCT03480152) was initiated in 2018 to determine whether the mRNA vaccine, National Cancer Institute (NCI)-4650, was safe and could shrink metastatic melanoma. It was terminated in 2020 due to slow accrual with only five patients enrolled.10
Are ANY Vaccines Approved for Melanoma?
Fortunately, yes. T-VEC (Imlygic) is an oncolytic virus therapy that treats advanced melanoma. This type of treatment vaccine is different, and it is not made up of mRNA personalized to the patient. Instead, oncolytic virotherapy introduces an engineered virus into melanoma cells, causing them to burst open. When this occurs, they spill out more viruses and pieces of the destroyed cancer cell.
Both the viruses and the cancer cell particles that came from the burst melanoma cell will be swallowed by other cells, slowly and sequentially ridding the body of the virus and cancer cells, as the process continues. The immune cells may also recognize the cancer cell particles on other cells and eliminate the infected cells. Learn more about this type of therapy on our Oncolytic Virus Therapy and How Can It Treat Melanoma information page.
Conclusion
The mRNA vaccines in active clinical trials for melanoma are specifically designed to treat the disease, rather than prevent it. The personalized mRNA vaccines are created after removing and analyzing the DNA expressed by an individual’s melanoma; the other vaccine targets melanoma antigens that many patients with melanoma will express. One vaccine for melanoma, T-VEC, is approved for use, although the design is based on using a virus to burst cells and does not rely on mRNA.
Although mRNA vaccines for melanoma represent an exciting concept, it may be some time before we know whether these are a breakthrough or a bust. Some preliminary results show promise, whereas some clinical trials for mRNA were terminated due to low accrual.
Nevertheless, the 2023 Nobel Prize winners in medicine were a research team investigating mRNA vaccine strategies. Years earlier, they figured out how to design mRNA for therapeutic benefit by communicating instructions to cells and the immune system. Companies applied their research in 2020 when the COVID-19 pandemic hit and developed vaccines against the virus. Hundreds of millions have been injected with this technology that allows your immune system to recognize and kill COVID-19— essentially, a vaccine with mRNA. We hope the same success can be replicated in melanoma.
References
- Yao R, Xie C, & Xia X. Recent progress in mRNA cancer vaccines. Hum Vaccin Immunother. 2024; 20(1):2307187. doi: 10.1080/21645515.2024.2307187.
- Weber JS, Khattak MA, Carlino MS et al. Individual neoantigen therapy mRNA-4157 (V940) plus pembrolizumab in resected melanoma: 3-year update from the mRNA-4157-P201 (KEYNOTE-942) trial. Presented at the 2024 American Society of Clinical Oncology. Abstract LBA9512.
- Hieken TJ & Ariyan C. Personalized mRNA Vaccines and Contemporary Melanoma Practice. Ann Surg Oncol. 2025;32(1):1-2. doi: 10.1245/s10434-024-15731-w.
- Clinicaltrials.gov. A Clinical Study of V940 Plus Pembrolizumab in People With High-Risk Melanoma (V940-001). Accessed 4.4.25. Updated 11.18.24. Available at: https://clinicaltrials.gov/study/NCT05933577.
- Clinicaltrials.gov. Trial With BNT111 and Cemiplimab in Combination or as Single Agents in Patients With Anti-PD-1-refractory/Relapsed, Unresectable Stage III or IV Melanoma. Accessed 4.3.25. Updated 3.25.25. Available at: https://clinicaltrials.gov/study/NCT04526899.
- Zak MM & Zangi L. Clinical development of therapeutic mRNA applications. Molecular Therapy. 2025. doi: https://doi.org/10.1016/j.ymthe.2025.03.034
- Clinicaltrials.gov. Phase 1/2 Study of Intratumoral Injection of STX-001 in Advanced Solid Tumors as Monotherapy or in Combination With Pembrolizumab. Accessed 4.4.25. Updated 2.11.25. Available at: https://clinicaltrials.gov/study/NCT06249048?term=NCT06249048.
- Gao W, Pan J & Pan J. Antitumor Activities of Interleukin-12 in Melanoma. Cancers (Basel). 2022;14(22):5592. doi: 10.3390/cancers14225592
- Sethna Z, Guasp P, Reiche C et al. RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer. Nature. 2025 Mar;639(8056):1042-1051. doi: 10.1038/s41586-024-08508-4.
- Clinicaltrials.gov. Messenger RNA (mRNA)-Based, Personalized Cancer Vaccine Against Neoantigens Expressed by the Autologous Cancer. Accessed 4.4.25. Updated 6.2.20. Available at: https://clinicaltrials.gov/study/NCT03480152.
Recent Posts

Update on the State of Vaccines for Melanoma

Healing Through Creativity: Art Therapy in Cancer Care

Side Effect Central: Lichen Planus Explained

Behavioral Addiction Responsible for Excessive Indoor Tanning


