Clinical Trials

What Are Clinical Trials?
Clinical trials are clinical research studies involving human participants. These trials evaluate how well an intervention—a drug, device, procedure, delivery system, strategy for behavioral change, etc.—works on humans. Clinical trials are essential to learning more about melanoma and other cancers and diseases and how to prevent or cure them.
The National Institutes of Health (NIH) has four specific criteria to determine whether a research study is a clinical trial. All four of these have to be true for a study to be a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
Researchers design clinical trials to answer specific questions and then must create and follow a specific plan called a protocol. They also determine details such as who will qualify to participate; how many patients will participate; how long the study will last; what data will be collected and how; and so on.
Different types of clinical trials have different goals.
- Behavioral trials evaluate or compare ways to promote behavioral changes designed to improve health.
- Diagnostic trials study or compare tests or procedures for diagnosing a particular disease or condition.
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning. Approaches may include medicines, vaccines, or lifestyle changes.
- Quality-of-life trials, or supportive care trials, explore and measure ways to improve the comfort and quality of life for people with conditions or illnesses.
- Screening trials test new ways for detecting diseases or health conditions.
- Treatment trials test new treatments, new combinations of medicines, or new approaches to surgery or radiation therapy.
Treatment trials are often what people think of when they refer to clinical trials, and they are understandably an important type of clinical trial for people diagnosed with cancer. These web pages will primarily focus on treatment trials.
Why Are Clinical Trials Important?
Cancer affects us all, whether we have it, care about someone who does, or worry about getting it in the future. Clinical trials contribute to our knowledge base and our progress fighting cancer. If a prevention intervention proves effective in a study, it may become widely recommended. If a new treatment proves effective in a study, it may soon become a new standard treatment that will help many patients. It’s important to remember that the current standard treatments for melanoma are available because of previous clinical trials. Clinical trials may also answer important scientific questions and suggest future research directions. Because of progress made through clinical trials—especially clinical trials in melanoma—many people are now living longer with a better quality of life.
What Happens in a Clinical Trial?
In a clinical trial, doctors carry out research to understand things such as the safety of a treatment; the appropriate dosage of a treatment; how the treatment affects the patients; and whether the desired results occur. In cancer clinical trials, patients often receive treatments that are new and innovative though not completely proven to work or be risk-free; however, the protocol is specific to each clinical trial. What happens in a clinical trial also depends on the phase of that clinical trial. There are four phases, and each type has different goals.
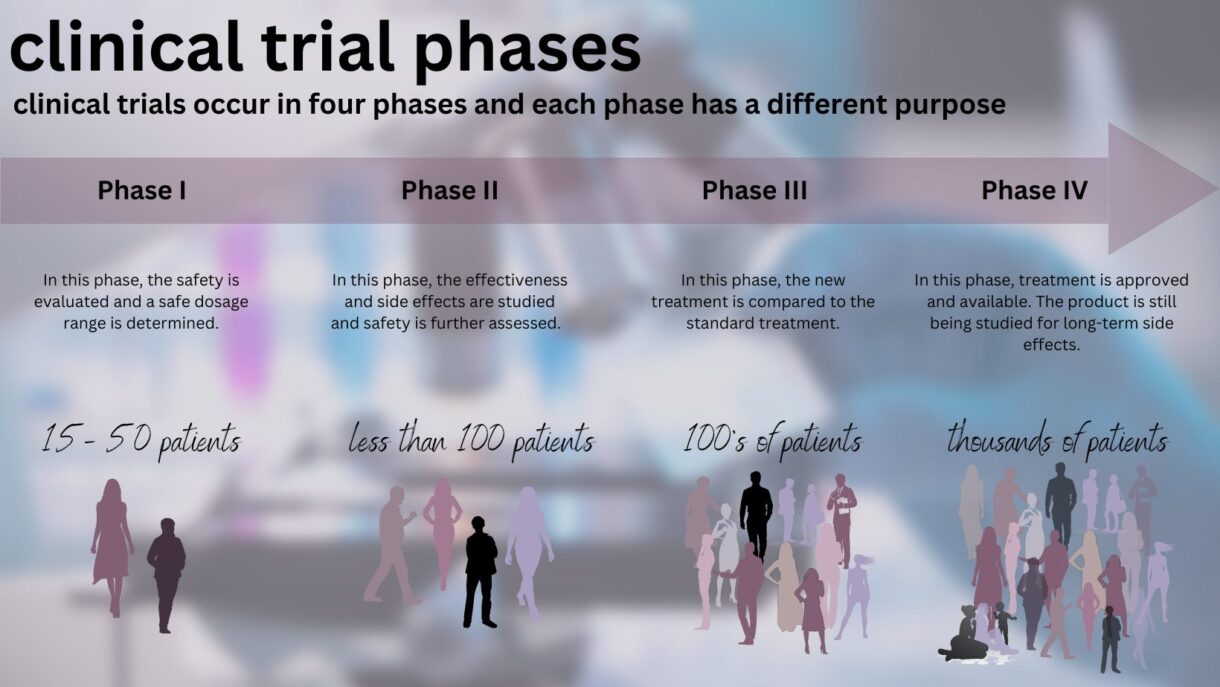
What are the Four Phases of Clinical Trials?
As a new treatment is evaluated, it goes through four phases, each with a different purpose:
- Phase I: Safety, administration, and dosage. Phase I trials can last for several months to several years and usually involve approximately 15-50 people. These trials test if a new treatment is safe, seek the best way to administer the treatment, and seek the appropriate dose of a treatment. The volunteers who participate are divided into small groups called cohorts. The first cohort is given the new drug at a certain dose. As long as the first group does not experience severe side effects or other issues, the second cohort is given a higher dose of the drug, and so on.
- Phase II: Effectiveness in a single cancer. Phase II trials typically involve about 100 patients and can last several months to several years. Many Phase II trials test if the new treatment works in a single type of cancer. Safety is also monitored in Phase II trials.
- Phase III: Better than standard of care? Phase III trials involve several hundred to several thousand patients across the country or world and can last as long as four years or even longer. They test if a new treatment is better than the standard treatment by randomly selecting patients to receive either the standard treatment or the new treatment. Phase III trials are what most people think of when they refer to clinical trials. If the results of the Phase III trial are sufficient for the FDA—if the drug is determined to provide benefits that outweigh its known and potential risks for the intended population—the treatment is approved and made available for all patients.
- Phase IV: Long-term side effects. Phase IV trials involve several thousand patients and study an FDA-approved drug’s long-term side effects.
When Should I Consider a Clinical Trial?
The best time to consider a clinical trial is each time you are faced with a treatment decision.
Where Do Clinical Trials Fit Into A Melanoma Treatment Plan?
Your goal is to find the best treatment available whenever you make a treatment decision. There may be a good standard of care for you—care that experts believe is appropriate for your specific melanoma diagnosis and treatment history.
Sometimes, though, the current standard of care is not as effective as you and your doctor would like. Sometimes the standard of care works for a while but then stops working. Sometimes there is no standard of care for your situation. At these times, participation in a clinical trial may be the best option for you.
How Do I Join A Clinical Trial?
The best way to find a clinical trial for you is to talk to your doctor. If you are being treated in an academic medical center or large cancer center, the discussion about participating in clinical trials usually takes place early in the process. If you are in a community cancer center or private practice setting, you may need to ask your doctor whether there are any clinical trials that are appropriate for you at this location or other locations. AIM at Melanoma also offers a service that connects patients to appropriate clinical trials. Important note: You may seek a clinical trial yourself and discuss it with your doctor; you do not have to be “invited” to participate in one.
Where Do I Receive Treatment in a Clinical Trial?
When you take part in a clinical trial, you receive your treatment in a cancer center, hospital, clinic, and/or doctor’s office that has qualified to become part of the study. These doctors and institutions must submit a rigorous protocol for approval before they may participate in a clinical trial. Doctors, nurses, social workers, and other health professionals may be part of your treatment team. They will follow your progress closely. You may have more tests and doctor visits than you would if you were not taking part in a study to gain more information on the medication and its effect on you. You will follow a treatment plan your doctor prescribes, and you may also have other responsibilities such as keeping a log or filling out forms about your health. Many studies continue to check on patients even after their treatment is over, and it is common to have follow-up visits well after treatment has stopped to reassess your health. With a new therapy or treatment, it may be some time before a complete understanding of the effects are realized.
What Happens When a Clinical Trial Ends?
Once a clinical trial ends, the researchers will carefully examine data collected during the study to decide the next steps. After a phase I or II trial, the researchers assess what they’ve learned about safety, side effects, and dosage to decide whether to proceed to the next phase. When a phase III trial is completed, the researchers (and the FDA) decide whether the results indicate that the treatment, procedure, or new medical approach was effective or not.
As a participant, you should be provided information before the study starts about how long it will last and how the end of the trial will affect you, such as whether the treatment will continue to be available to you. Ask questions whenever you have them. And, importantly, patients can choose to discontinue their participation in a trial at any time but should discuss any concerns or questions with their doctor.
Ask a Melanoma Expert
AIM at Melanoma knows a melanoma diagnosis can be confusing, but you don’t have to handle it alone. That’s why our team includes a specialized physician assistant to help you navigate through the entire spectrum of care. Our melanoma medical expert, Melissa Wilson, PA-C, MPAS, can provide accurate answers to a wide range of melanoma questions on a confidential basis.
Contact Melissa HERE